Shallow Water Habitats
Ecosystem Processes - Nutrient Cycling
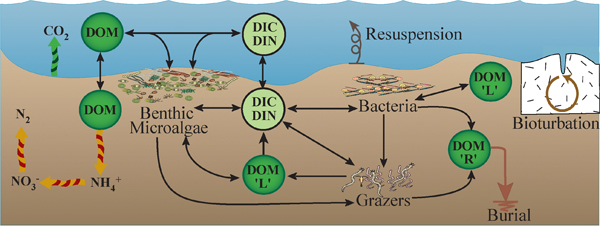 Many different nutrients are required by living organisms. These nutrients are cycled through the food web, while organic matter is being produced or degraded. Nitrogen
is an important macronutrient, which gets a great deal of attention
from estuarine scientists and resource managers because nitrogen
enrichment of estuaries and the coastal zone plays a major role in
anthropogenic (human-induced) eutrophication.
Many different nutrients are required by living organisms. These nutrients are cycled through the food web, while organic matter is being produced or degraded. Nitrogen
is an important macronutrient, which gets a great deal of attention
from estuarine scientists and resource managers because nitrogen
enrichment of estuaries and the coastal zone plays a major role in
anthropogenic (human-induced) eutrophication.
Organisms require nitrogen to form essential compounds such as amino
acids, proteins, DNA, RNA, and chlorophyll. In many coastal
environments nitrogen limits growth of phytoplankton and some nuisance
primary producers such as macroalgae. Agricultural land use is a major
source of nitrogen to the Chesapeake Bay watershed and many other
estuarine and coastal ecosystems. Other important sources include
atmospheric deposition and groundwater. When marine and estuarine
ecosystems are over-enriched with nitrogen, blooms of phytoplankton or
macroalgae may occur, which contribute to eutrophication.
Nitrogen occurs naturally in the environment in various forms,
including inorganic species, such as ammonium (NH4+), nitrate (NO3-),
nitrite (NO2-), and nitrogen gas (N2) and organic forms such as amino
acids, proteins, DNA and RNA.
Microbes, such as bacteria, are the primary mediators of nitrogen
transformations, converting inorganic nitrogen into a variety of other
inorganic or organic species. A wide variety of bacteria and some
Archaea are also responsible for converting nitrogen gas, which is
unavailable to all other forms of life, to ammonium, which can support
growth by most living organisms, a process called nitrogen fixation.
Bacteria may remove inorganic nitrogen from the environment by
denitrification or transform nitrogen into bacterial products, which
are less available to other living organisms, and which may accumulate
in the ecosystem. Primary producers also convert inorganic nitrogen
into organic matter.
Key nitrogen transformation processes are remineralization,
nitrification, denitrification, anammox, dissimilatory nitrate
reduction to ammonium, assimilation, and nitrogen fixation.
Remineralization
(or, simply, mineralization) is the breakdown of protein and other
organic matter by bacteria during respiration, which then releases
ammonium and phosphate. These are inorganic (mineral) forms.
Denitrification
is a microbially mediated anaerobic process that converts nitrate to
nitrogen gas as the final product, thereby removing it from the system
to the atmosphere. Denitrifiers use nitrate (which contains oxygen),
rather than free oxygen, to release energy from organic matter during
the process of oxidation.
Primary producers,
such as plants, trees, phytoplankton, and benthic micro- and
macroalgae, as well as bacteria and archaea take up (in a process
called assimilation) inorganic nitrogen for organic matter and biomass production.
Anammox is an anaerobic process by which bacteria ich results in the removal of nitrogen from the estuarine ecosystem.
Dissimilatory nitrate reduction to ammonium is an anaerobic process in which bacteria reduce nitrate to ammonium, a process which competes with denitrification and retains nitrogen in the ecosystem. When sulfide concentrations are high in anoxic sediment, dissimilatory nitrate reduction to ammonium is favored over denitrification.
Reducing nitrogen gas to ammonium, which is mediated by a large and diverse group of bacteria and archaea, including cyanobacteria. Nitrogen fixers make nitrogen biologically available to organisms in terrestrial and aquatic environments.For further information about nutrient cycling in estuarine and coastal marine habitats, refer to the following:
Anderson, I.C., McGlathery, K. J., and Tyler, A. C. (2003). Microbial mediation of reactive nitrogen transformations in a temperate lagoon. Mar. Ecol. Prog. Ser. 246:73-84.
Joye, S. B. and I. Anderson, 2007. Nitrogen cycling in Estuarine and Nearshore Sediments. In: Capone, D., Bronk, D., Carpenter, E. and Mulhollond, M. (Eds), Nitrogen in the Marine Environment, Springer Verlag, in press.
Mann, K. H.
2000. Ecology of coastal waters, with implications for management.
Blackwell Publishing; Chapters 2 -7 have relevant sections on nutrients
and their effects.
Munn, C. B. 2004. Marine Microbiology: Ecology and Applications. Garland Science/BIOS Scientific Publishers.
OzCoast and OzEstuaries website: http://www.ozcoasts.org.au/indicators/water_column_nutrients.jsp
http://www.ozcoasts.org.au/conceptual_mods/index.jsp
Valiela, I. 1995. Marine Ecological Processes (2nd Edition). Springer; Chapter 14 – Nutrient cycles and ecosystem stoichiometry.

