Gulf Spill FAQs
- Will Gulf oil reach Virginia?
- How might any Gulf oil impact Virginia?
- What is crude oil?
- How does oil affect marine plants and animals?
- What biological and physical processes act to degrade oil?
- What effect might the oil have on the Gulf of Mexico dead zone?
- What are the pros & cons of various clean-up techniques?
- How does Virginia deal with oil spills in its own waters?
- Could a similar spill occur off Virginia's coastline?
- What role does VIMS play in the oil-spill response?
1. What is the likelihood that oil from the Gulf spill could reach Virginia's Atlantic coastline or Chesapeake Bay?
As shown in this computer simulation from the National Center for Atmospheric Research (NCAR), it is relatively unlikely that Virginia will see much oil from the present Gulf spill, as the northward-flowing Gulf Stream turns northeast at Cape Hatteras, away from Virginia. However, an eddy or "ring" could spin off the northwest side of the Gulf Stream and be carried toward the Virginia shore by the gentle current that flows southward between the Gulf Stream and the mid-Atlantic shore. The greatest likelihood of oil reaching Virginia's Atlantic coastline would occur if strong winds from the east or northeast were to act on a Gulf Stream 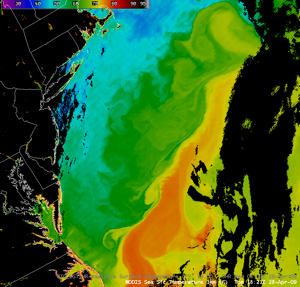 eddy containing relatively high concentrations of oil. Transport of coastal waters into Chesapeake Bay does occur, but it would take an unusual and atypical combination of circumstances for a meaningful quantity of material from the Deepwater Horizon spill to make it into the Bay.
eddy containing relatively high concentrations of oil. Transport of coastal waters into Chesapeake Bay does occur, but it would take an unusual and atypical combination of circumstances for a meaningful quantity of material from the Deepwater Horizon spill to make it into the Bay.
2. If oil from the Gulf spill were to reach Virginia's Atlantic coastline or Chesapeake Bay, what might be some of the potential impacts?
Although the weathering profile of the crude oil spilled from the Deepwater Horizon platform is not fully known, any oil that might make it to Virginia would most likely be in the form of individual tar balls, as most of the volatiles would have evaporated or been otherwise weathered.
Potential impacts and clean-up would depend on the nature of the oil residue and the nature of the impacted shoreline. Salt marsh clean-up is difficult and must consider the harm done to the marsh by walking or driving on it in order to clean it. If marsh plants are coated with oil, the surface portion of the plant will die, but the root might remain viable. In some circumstances, if the polluting material is widespread and readily flammable, burning the marsh is a good option. The fire consumes both the marsh grass and hydrocarbons, but leaves the root system intact so the marsh can regrow. One consideration in burning is the resulting air pollution. "Cleaning" a marsh with dispersants runs the risk that the small droplets of dispersed oil will be carried down into the marsh soil, killing the plant roots.
Most of Virginia's ocean shoreline is sandy. On sandy shores, tar balls can be picked up or collected by scraping the entire surface of the beach. More liquid pollutants can be washed off the beach with dispersants (again with concerns about the consequences of the dispersant) or collected by scraping the surface sediment. The type of clean-up seen after the Exxon Valdez oil spill—involving high-pressure water hoses to wash oil off of rock—would be unlikely to take place in Virginia due to rarity of natural rocky shorelines. [top]
3. What is crude oil?
Crude oil is a naturally occurring mixture of hydrocarbons of various molecular weights. It can also contain varying amounts of nitrogen, oxygen, and sulfur, and trace amounts of metals such as iron, nickel, copper, and vanadium. Crude oil varies greatly in appearance depending on its composition. It is usually black or dark brown, although it may be yellowish or even greenish.
Under the pressure and temperature conditions of the Earth's surface, the lighter hydrocarbons methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10) occur as gases, while the heavier ones, including pentane (C5H12), hexane (C6H14), and heptane (C7H16), may be liquids or solids. The proportion of gas or liquid hydrocarbons in underground oil reservoirs depends on the subsurface conditions.
The proportion of light hydrocarbons in crude oil varies greatly between different oil fields and ranges from as much as 97% by weight in the lighter oils to as little as 50% in the heavier oils and bitumens. The South Louisiana crude in the Gulf spill is considered a "light" crude oil and is known to evaporate and degrade at a faster rate than heavier weight oils from other locations.
Crude oil is typically less dense than seawater, so any oil released into the marine environment typically rises to or stays on the water surface as a thin layer, or slick. Through time, evaporation of the lighter, volatile components of a surface oil slick will tend to leave the heavier components behind as tar balls. [top]
4. How does oil affect marine plants and animals?
Crude oil is a mixture of simple and complex hydrocarbons such as methane, butane, and hexane that are toxic to living things when ingested or absorbed in sufficient quantities. Crude oil can also contain other toxic materials such as heavy metals.
Crude oil can harm marine plants by coating their surfaces, thus blocking the sunlight needed for photosynthesis. Physical coating of marine animals can also produce harm through absorption of toxic materials through the skin and by physical damage to feathers, gills, and other sensitive tissues and organs. Physical coating is particularly prevalent among organisms that spend considerable time on the surface, such as birds, sea turtles, and marine mammals; and is particularly injurious to plankton, larvae, and other microscopic organisms with relatively large surface areas. [top]
5. What biological and physical processes act to degrade oil?
Crude oil released into seawater through spills or naturally occurring seeps can be degraded by physical, chemical, and biological processes. The extent of degradation depends on the oil's initial composition and the environmental setting.
Light crude oils contain relatively high proportions of volatile components that readily evaporate from surface slicks, especially during the heat of summer. Oils can also disperse in water through turbulent mixing to form an emulsion known as "mousse" or can adhere to suspended particulate matter. Little is known of degradation rates in dark subsurface environments where low temperatures prevail.
Sunlight, especially the UV portion of its spectrum, can play an important role in the breakdown of oil. Some studies have shown that photo-products tend to be more toxic than parent compounds in the crude oil.
In marine environments, bacteria play an important role in the biodegradation of oil. The extent of degradation is affected by both the chemical structure of each crude-oil constituent and the availability of dissolved nutrients since crude oil has little or no nitrogen or phosphorous in forms that are available to living organisms.
Bacterial degradation is most effective when oil is dispersed into small particles, which provide a large surface area for colonization and metabolic interactions. Dispersants tend to accelerate biodegradation for this reason but some such as Corexit are also toxic to animals and plants.
Bacteria initially tend to degrade the most metabolically favorable linear alkanes and some simpler aromatic constituents. The degradation of weathered oil, containing a variety of ring structures such as the water-insoluble asphaltenes and other large-molecular-weight heterocycles is not well understood.
Gradual loss of volatile and dissolved compounds from crude oil leads to the formation of tar balls that may remain at the water surface or coat any available surface. These can be rafted inshore by winds or tides.
Fungi play an important role in the biodegradation of crude oil in inshore habitats such as marshes. If oil becomes buried in oxygen-poor marsh sediments it may persist for very long periods of time. This is because the most efficient metabolic pathways of oil degradation by microorganisms require oxygen. [top]
6. What effect might the oil have on the Gulf of Mexico dead zone?
A large and persistent low-oxygen "dead zone" affects the waters of the Gulf of Mexico adjacent to the Mississippi Delta. This and other dead zones form when excess nutrients, primarily nitrogen and phosphorus, enter coastal waters and help fertilize blooms of algae. When these algae die and sink to the bottom, they provide a rich food source for bacteria, which in the act of decomposition consume dissolved oxygen from surrounding waters. Excess nutrients in the Louisiana Gulf region are supplied by the outflow of the Mississippi River. Scientists are now actively investigating whether bacterial degradation of the oil from the Gulf spill may contribute to low-oxygen conditions in the region. [top]
7. What are the pros & cons of various clean-up techniques?
Each of the three main techniques for cleaning up a marine oil spill—collection and removal, dispersal, and burning—has its own benefits and drawbacks.
Collection of oil through the use of containment booms and skimmers is an effective way to gather and extract oil from water. Booms are particularly effective at preventing oil from entering marine areas with restricted openings to the open ocean, such as barrier-island lagoons. Another benefit is that the recovered oil can subsequently re-enter the energy stream. However, use of these devices is restricted to situations in which an oil slick is concentrated in a relatively small area.
Chemical dispersants work by breaking the oil within a surface slick into much smaller globules. Dispersing a surface oil slick can benefit the environment by preventing the slick from encountering and coating marine organisms (e.g., sea turtles, marine mammals, birds) or sensitive habitats (e.g., marshes, sandy or rocky beaches). Dispersants can also speed biological degradation of oil by increasing the surface area available for bacteria and other micro-organisms. However, significant questions remain regarding the potential environmental effects of dispersed oil on smaller marine organisms such as plankton and larvae, and on the potential toxicity of the dispersant chemicals themselves.
Burning of spilled oil is an effective means of removing toxic hydrocarbons from the ocean surface, and can be a preferred, low-impact method for removing oil from marshes (marsh grasses will regrow from roots following burning, whereas physical collection of oil from marshes involves the use of heavy machinery that can cause long-term damage to the marsh habitat). The primary drawback to burning is the resulting damage to air quality. [top]
8. How is Virginia positioned to deal with oil spills in Chesapeake Bay or in Virginia's coastal waters?
The federal government, specifically the US Coast Guard, is the lead agency for oil spills in U.S. waters. The Virginia Department of Emergency Management (VDEM) is the lead agency for the Commonwealth. The Virginia Department of Environmental Quality (DEQ), the Virginia Marine Resources Commission (VMRC), and the Virginia Institute of Marine Science (VIMS) also would play important roles. [top]
9. How does the Deepwater Horizon platform and drilling environment compare to the drilling that might take place off Virginia's coastline?
The geologic and oceanographic settings of the Deepwater Horizon platform and the potential lease sale off the Virginia coast are similar. Both are in ~5,000 ft of water and lie about 50 miles off the coast. (Although the Virginia's proposed [now on hold] offshore lease area begins 50 miles from the shore, any wells likely would be farther away.)
The chemical character of the Gulf oil cannot currently be compared to any oil or gas that would be produced off of Virginia, as the composition of the latter is as yet unknown.
The greatest difference is in the nature of the adjacent shorelines—the marshes of the Mississippi Delta are open to the Gulf, whereas Virginia's salt marshes are either within Chesapeake Bay or behind the barrier islands of the Delmarva Peninsula. If the Eastern Shore were threatened, a substantial effort would be needed to keep the oil from entering the inlets that separate the barrier islands. [top]
10. What role would the Virginia Institute of Marine Science (VIMS) play if oil were to reach local waters?
VIMS' primary role in responding to an oil spill (whether local or from afar) would be to
- provide advice and information on the likely movements of the material
- help set priorities for identifying the areas that are in greatest need of protection, and
- provide information on toxicity.
VIMS' primary role is not as a clean-up agency—though it is probable that the Institute would be called on to assist other state and federal agencies with scientific expertise and logistical aid from our vessel fleet. Members of the VIMS community would also be likely to volunteer to assist where needed.
VIMS has also played a key role in collecting and analyzing scientific data regarding the environmental effects of exploring for and possibly extracting oil and gas from Virginia's continental shelf. VIMS researchers began their studies in the late-1970s following the 1973 oil crisis:
Rooney-Char, A. H. and R. P. Ayres (1978). Offshore pipeline corridors and landfalls in coastal Virginia. Special Report in Applied Marine Science and Ocean Engineering. Report 190. Gloucester Point, Virginia Institute of Marine Science.
Volume 1: Offshore pipeline corridors and landfalls in coastal Virginia
Volume 2: Appendices
Burreson, E. M., D. F. Boesch, et al. (1979). Middle Atlantic outer continental shelf environmental studies. Special Report in Applied Marine Science and Ocean Engineering. Reports 191-204. Gloucester Point, Virginia Institute of Marine Science
Volume 1: Executive Summary
Volume 2A: Chemical and Biological Benchmark Studies: Physical Climatology, Oceanography, and Zooplankton
Volume 2B: Chemical and Biological Benchmark Studies: Benthic Ecology
Volume 2C: Chemical and Biological Benchmark Studies: Fish Communities and Bacteriology
Volume 2D: Chemical and Biological Benchmark Studies: Histopathology, Trace Metals, Hydrocarbons, and Wave Climate
Volume 3: Geologic Studies
[top]
