Jellyfish
Climate-related factors which might influence Chrysaora quinquecirrha distributions are:
- Water Temperature predicted to increase due to air temperature increase
- Salinity predicted to increase in some rivers due to sea level rise, also may seasonally decrease due to increasing storm activity
- Stream Flow may increase or decrease depending on storm activity
- Zooplankton (food source) abundance, which is reliant on phytoplankton bloom timing, location and abundance
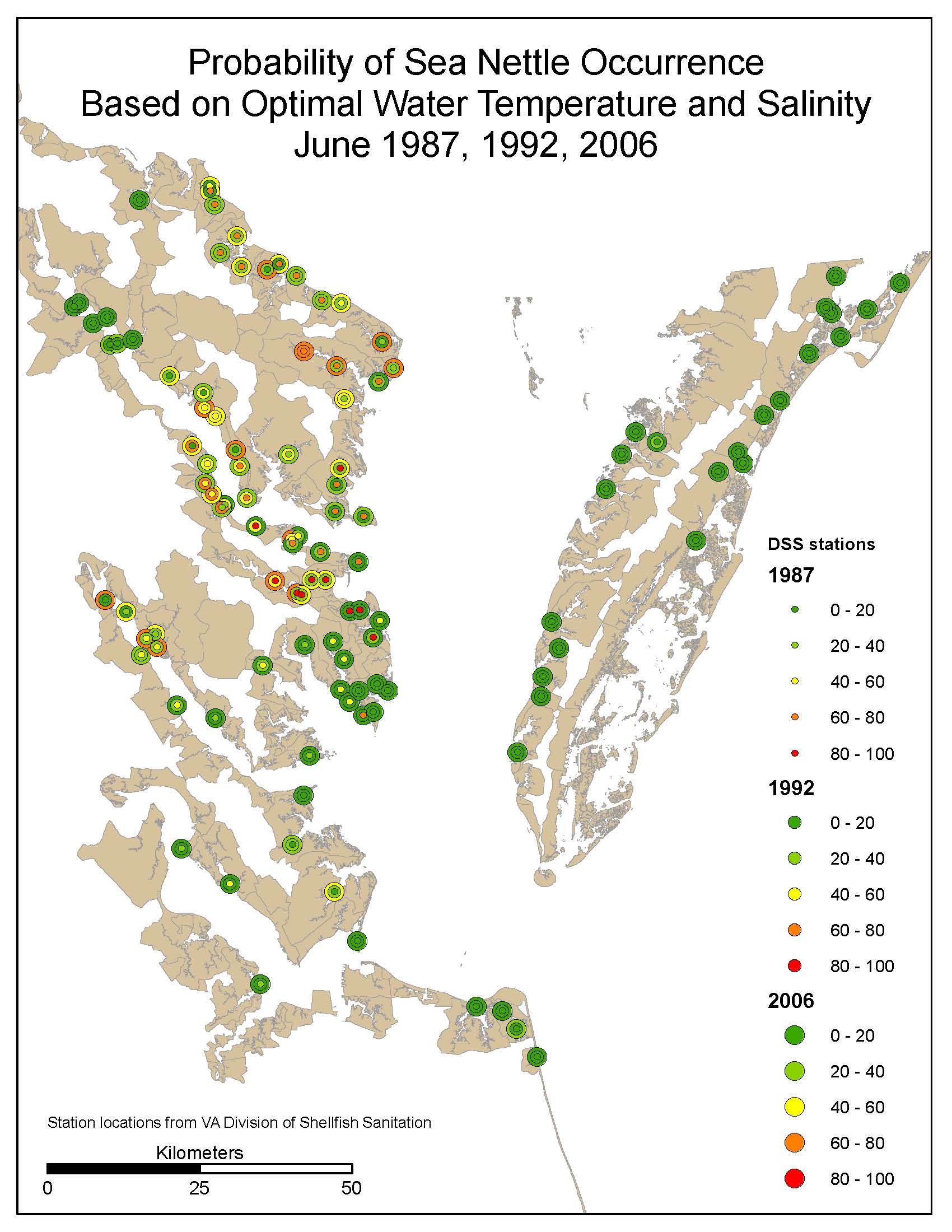
Jellyfish medusae are found within a narrow range of water temperatures (26 – 30 C) and salinities (10 – 16 ppt). The occurrence of these conditions is a good predictor of the presence of jellyfish (Decker et al. 2007). In some areas, there is evidence that jellyfish populations are increasing in response to changing climate (Brodeur et al. 1999, Mills 2001). See the NOAA sea-nettle forcasting page for an interactive jellyfish habitat model built on salinity and temperature distributions.
Streamflow into the Chesapeake Bay, from January-June explains about 2/3 of the variation in jellyfish counts in Solomons, MD (Cargo and King 1990). During wet years, medusae appear late in the season in the mainstem of the Bay. In normal and dry years, the medusae appear earlier in the year and are found in the Bay tributaries (Decker et al. 2007). Medusae are produced in shallow water at approximately 17 C, while the upper lethal temperature is 34 C (Cargo and Schultz 1967).
Jellyfish blooms control plankton dynamics in coastal habitats through predation (Purcell et al. 2001). Increasing jellyfish populations could cascade through the food chain, reducing zooplankton populations and changing the path of nutrient transformation. Jellyfish consume significant numbers of copepods (Purcell et al. 1992) and comb jellies (Purcell and Cowan 1995, Purcell and Decker 2005). Comb jellies eat larval oysters, while jellyfish cannot digest them; by reducing comb jelly abundance, jellyfish may help increase oyster populations (Purcell, web communication).
Jellyfish consume Anchoa mitchelli eggs and larve, and compete for food with the adults (Purcell et al. 1994, Purcell 1992). Anchoa mitchelli are an important food source for striped bass and bluefish, allowing changes in jellyfish abundances to translate up the food chain, and ultimately impact fisheries.
Other potential relationships which could be affected by climate include:
Potential Relationships |
Data Available? |
Data Sets |
| Changes in streamflow and sea nettle population? | Yes |
|
| Changes in precipitation and sea nettle population? | Yes |
|
| Increased nutrients producing excess food leading to increased sea nettle population? | Yes |
|
| Relationship between land development and sea nettle distribution? | Partial |
|
| Impact of sea nettle population on bay anchovy population? | Yes |
|
| Impact of sea nettle population on bay anchovy predators (striped sea bass, bluefish)? | Yes |
|
| Impact of sea nettle population on copepod population and distribution? | Yes |
|
| Impact of sea nettle population on ctenophore population and distribution? | Yes |
|
ReferencesBrodeur, R., C. Mills, J. Overland, G. Walters, and J. Schumacher (1999) Evidence for a substantial increase in gelatinous zooplankton in the Bering Sea, with possible links to climate change. Fish Oceanography 8:296-306. Cargo, D. and D. King (1990) Forecasting the abundance of the sea nettle, Chrysaora quinquecirrha, in the Chesapeake Bay. Estuaries 13:486-491. Cargo, D. and L. Shultz (1966) Notes on the biology of the sea nettle, Chrysaora quinquecirrha, in the Chesapeake Bay. Chesapeake Science 8:209-220. Mills, C. (2001) Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobilogia 451:55-68. Purcell, J., web communication: Purcell, J. (1992) Effects of predation by the scyphomedusan Chrysaora quinquecirrha on zooplankton populations in the Chesapeake Bay, USA. Marine Ecological Progress Series 87:65-76. Purcell, J. (2005) Climate effects on formation of jellyfish and ctenophore blooms. Journal of the Marine Biological Association UK 85:461-476. Purcell, J. and J. Cowan, Jr. (1995) Predation by the scyphomedusan Chrysaora quinquecirrha on Mnemiopsis leidyi ctenophores. Marine Ecological Progress Series 129:63-70. Purcell, J., D. Nemazie, S. Dorsey, E. Houde and J. Gamble (1994) Predation mortality of bay anchovy Anchoa mitchelli eggs and larves due to scyphomedusae and ctenophores in the Chesapeake Bay. Marine Ecological Progress Series 114:47-58. |

