Hematodinium Species
Parasitic Dinoflagellates of Marine Crustacea
Introduction

Parasitic dinoflagellates in the genus Hematodinium are important parasites of marine Crustacea. Outbreaks of these parasites have damaged commercial stocks of Norway lobster (Nephrops gicus), snow crab (Chionoecetes opilio), Tanner crab (Chionoecetes ), American blue crab (Callinectes), and velvet swimming crab (Necora puber). Species of Hematodinium can reach high-enough levels to regulate their host populations, but mortalities are also based on the un-fished juveniles and females, hosts not normally sampled by fisheries; hence impacts are often underreported. Seasonal prevalences of up to 85% occur annually in many host populations, in effect, these parasites form "silent" blooms in the water column with many crabs and other crustaceans at risk of disease
There are two described species of Hematodinium. The type species H. perezi was originally described from Carcinus maenas and Liocarcinus depurator from France (Chatton & Poisson 1931). It has since been documented in blue crabs from the USA (Newman & Johnson 1975, Messick & Shields 2000). A second species, H. australis, was described from the Australian sand crab, Portunus pelagicus (Hudson & Shields 1994). Hereafter we refer to the species in Callinectes sapidus as H. perezi. Infections in other hosts have not been adequately described.

Hematodinium is one of the most economically significant diseases of marine decapod Crustacea. In Alaska and Newfoundland, fisheries for the Tanner crab (Chionoecetes bairdi) and snow crab (C. opilio) have experienced outbreaks of Hematodinium, which causes a condition known as "Bitter Crab Disease" (BCD) or “Bitter Crab Syndrome” due to the bitter aspirin-like flavor of infected crabs (Meyers et al. 1987, 1990, 1996, Taylor & Khan 1995). The disease infects a third of the commercial fishery for C. bairdi in southeast Alaska (Meyers et al. 1990). In the coastal snow crab fishery off Newfoundland, the disease has blossomed from a low of 0.037% in the early 1990s to 10% - 26% in 2000 (Pestal et al. 2003, Shields et al. 2005). In Virginia where the fishery for the blue crab undergoes periodic spring and autumn mortalities due to H. perezi (Messick & Shields 2000), losses to the fishery may exceed $500,000 per year in non-epidemic years (Stentiford & Shields 2005). Similar outbreaks have been reported in Georgia, particularly in relation to drought (Lee & Frischer 2004). In France, the fishery for the velvet crab (N. puber) suffered a catastrophic decline (>96 %) due to Hematodinium with a virtual loss of this fishery (Wilhelm & Mialhe 1996). Populations of the commercially valuable spider crab (Maia squinado) and edible rock crab (Cancer pagurus) also harbor infections of this parasite (Latrouite et al. 1988; Stentiford et al. 2002). In Scotland, the fishery for the Norway lobster (N. norvegicus) loses £2-4 million annually to Hematodinium, with prevalences ranging from 20% to 70% (Field et al. 1992, 1998, Stentiford et al. 2001c). Altogether, the reported declines in crabs attributable to Hematodinium are staggering.
However, the real cost of outbreaks of Hematodinium are hard to assess. Background mortalities due to the disease are difficult to determine because dead hosts quickly sink or rapidly become undiagnosable. Furthermore, infections occur primarily in juveniles and females and often go unnoticed by fishermen who target large market-sized animals that are typically adult males (Messick & Shields 2000, Shields 2003, Shields et al. 2005).
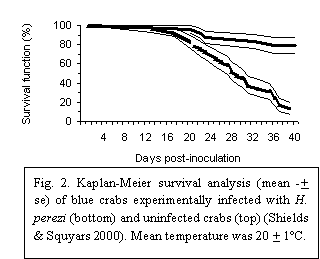
Hematodinium infections are highly pathogenic. In naturally and experimentally infected blue crabs, mortality was 87% over 40 days (Fig. 2) (Messick & Shields 2000, Shields & Squyars 2000, Messick et al. 2005). Interestingly, during challenge studies, small numbers of blue crabs were refractory to infection; these “immune” crabs exhibited significant increases in granulocytes, while not developing pathology or morbidity (Shields & Squyars 2000). A few other "immune" crabs that were serially challenged with infectious doses of H. perezi did not develop infections (Shields, unpub. data). Such ‘immunity’ in previously challenged hosts is an interesting phenomenon, particularly in light of the fact that mature hosts appear more resistant to infection compared to their juvenile counterparts (Messick 1994).
Susceptibility to many diseases varies with age- or size-specific changes in immune response, diet, or habitat use. Similarly, H. perezi infects small (< 30 mm CL) blue crabs significantly more than larger crabs (Messick 1994; Messick & Shields 2000). Juveniles may be more susceptible because they molt more frequently than adults and either obtain the disease at ecdysis (molting), undergo more stress because of molting, or have diet preferences that expose them to disease (Shields 1994).
 Seasonality has emerged as a significant epidemiological feature of all Hematodinium-host systems studied to date. In the coastal bays of Maryland and Virginia, prevalence shows regular sharp peaks in late autumn with a rapid decline in winter followed by moderate increases in late spring (Messick & Shields 2000; Sheppard et al. 2003). Epizootics can reach 100% prevalence during outbreaks (Messick 1994) with most of the diseased crabs likely dying of the infection (Messick & Shields 2000, Shields & Squyars 2000). Other Hematodinium infections show strong seasonality but the patterns differ (see Stentiford & Shields 2005); yet, in all of these systems, a nadir occurs when infections are extremely low or even undetectable in host populations. These nadirs are suggestive of either a latency of infection or an external reservoir for these parasites.
Seasonality has emerged as a significant epidemiological feature of all Hematodinium-host systems studied to date. In the coastal bays of Maryland and Virginia, prevalence shows regular sharp peaks in late autumn with a rapid decline in winter followed by moderate increases in late spring (Messick & Shields 2000; Sheppard et al. 2003). Epizootics can reach 100% prevalence during outbreaks (Messick 1994) with most of the diseased crabs likely dying of the infection (Messick & Shields 2000, Shields & Squyars 2000). Other Hematodinium infections show strong seasonality but the patterns differ (see Stentiford & Shields 2005); yet, in all of these systems, a nadir occurs when infections are extremely low or even undetectable in host populations. These nadirs are suggestive of either a latency of infection or an external reservoir for these parasites.
Click here to read more about our current project studying the effects of Hematodinium on the blue crab, Callinectes sapidus, population on the Eastern Shore of Virginia.
A complete list of Parasitic Dinoflagellates of Crustacea is available as well as a review of the subject (Shields, 1994)
Principal Investigator: Dr. Jeffrey Shields
